Easy on the Stomach Medications to Treat Thyroid Conditions
Introduction
Levothyroxine sodium monotherapy is ordinarily prescribed as treatment in replacement mode for hypothyroid patients worldwide (1). The need for an individually tailored dose has been strongly suggested (2). However, a meaning number of patients fail to show a biochemical and/or clinical response and larger doses of thyroxine are required to reach the target serum TSH concentrations (iii). Long-term suboptimal treatments have detrimental furnishings on trunk homeostasis (4). Frequent changes of dose and repeated diagnostic procedures in these patients have been related to incremental health costs (5). The causes of an increased thyroxine requirement take been recently reviewed (vi). Among these, the role of the altered gastric physiology on the subsequent intestinal T4 absorption has been repeatedly highlighted (7–ix). The mechanism by which intestinal absorption of thyroxine is dumb in patients with gastric disorders is unclear simply seems to pertain to the chemical and concrete properties of both naïve and salificated thyroxine molecule (10). Levothyroxine, the levo-isomer of thyroxine, is insoluble in water and in other usual organic solvents (xi). The salification process by a saturating excess of sodium hydroxide leads to the sodium common salt production that is the compound used in every pharmaceutical grooming of thyroxine (12). The oral is the preferred route of administration, due to safety and patients' preference (13). Oral levothyroxine assimilation is incomplete with reported percentages of virtually lxx% of the administered dose (14). The actual site of absorption is represented by the jejuno-ileal tract while only a few function of oral thyroxine is captivated in the duodenum (fourteen). Unlike the rat, no absorption in the large bowel has been described in humans (15). Furthermore, the study of the lag time between thyroxine ingestion and its appearance in the plasma excludes the possibility of absorption occurring in the tummy (15). However, several clinical studies suggested that the variations of gastric physiology might have a deep impact on oral thyroxine bioavailability, leading to an increased need for the drug. We aimed at reviewing the known and unknown on the role of the gastric surround in the absorption of oral thyroxine.
Gastric Contribution to Drugs Bioavailability
Well-nigh of the drugs are captivated at abdominal level. This assumption is based on its large surface extension and on the presence of dissimilar transporters on mucosal membrane (16). Absorption by duodenal mucosa is in plow regulated past its integrity, motility, mucus composition, and resident microbial population (16). On the contrary, drugs absorption from the stomach is unremarkably thought to exist negligible, although a passive improvidence through the gastric wall has been hypothesized and proven for compounds such every bit ethanol and small neutral molecules (xvi). The gastric absorption seems to be related to the ionization status of the drug that, in plough, depends on the gastric juice pH: in fact, in an acidic environs, acidic drugs are mainly nowadays and absorbed in a unionized form, being this process negligible for basic drugs (17). Yet, because of the paucity of papers on this topic farther studies are warranted. Anyway, the gastric environment exerts profound effects on drugs behavior and pharmacokinetic. In fact, several steps must be taken into business relationship when analyzing the so-called "gastric phase," which represents a pivotal prerequisite for the intestinal drug absorption (16). Once reached the tummy, the drug undergoes disintegration, dissolution, and possible atmospheric precipitation; furthermore, the active ingredient must accomplish the actual site of absorption. Disintegration causes the release of the active ingredient from the solid form. The elapsing of this stride is highly affected past the type of the formulation and past the excipients used (tablets, capsules, immediate-release formulations), the fasted or fed state, the gastric residence fourth dimension, and the gastric motor function (sixteen, eighteen). Simultaneously, the dissolution of the drug occurs. The dissolution process consists in the release of solute molecules from the solid phase to the liquid one, represented by the gastric juice. Once again, this process may exist affected by physicochemical characteristics of the drug (e.one thousand. particle size and polymorphisms) and by physicochemical conditions (19) on which the role of gastric juice pH and viscosity stand out (20). Several drugs (21), including levothyroxine, share these processes (Figure ane).
FIGURE i
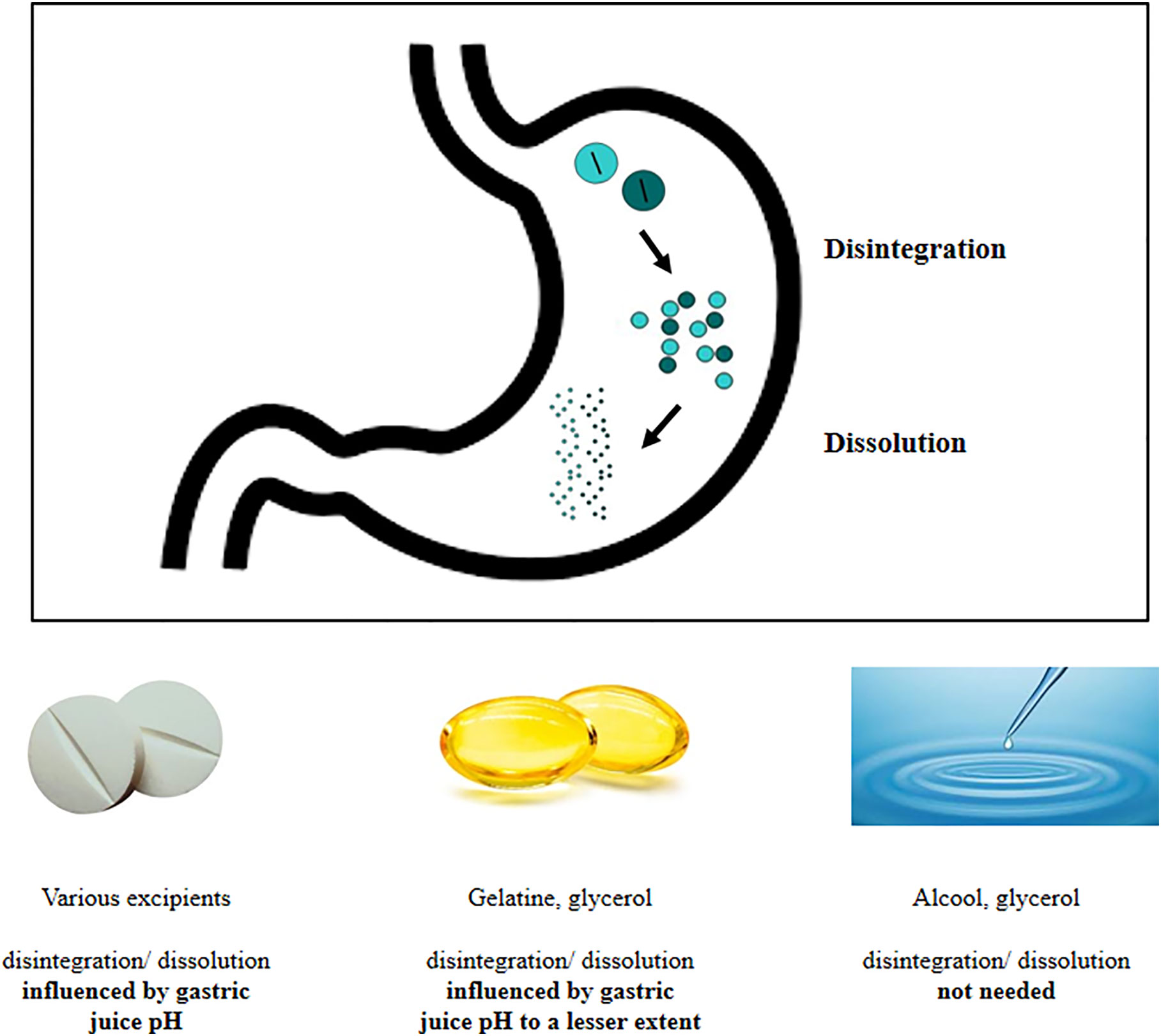
Figure 1 Delivery of agile ingredient at gastric level: behavior of different thyroxine formulations.
Levothyroxine Construction
The shared characteristic of all thyroid hormones is the thyronine nucleus, a diphenyl ether in which the two planar phenyl groups are oriented at an angle of 120 degrees (10). Four iodine substituents at the three,5,3′ and five′ positions and the presence of 4′ hydroxyl group in the outer ring characterize thyroxine molecule. Interestingly, the inner band contains an alanine side chain, which, at physiologic pH, is usually zwitterionic (i.due east., net positive charge at the amine group and net negative charge at carboxylic oxygen atoms). Thus, in the thyroxine molecule, three ionizable moieties exist, two acidic (the carboxylic and the phenolic one) and ane basic aminic group possessing 3 distinct pKa (10). It follows that thyroxine may be in four different ionization status such every bit zwitterionic, predominant in the range of pH between two.46 and vi.91 every bit well every bit cationic, anionic, and dianionic predominating at more farthermost pH (6, 11) depending on environmental pH. The nearly mutual pharmaceutical form is the pentahydrated sodium salt of T4 (22). Mondal et al. (23) have shown that almost two polymorphs of levothyroxine do be. These authors proved the being of two crystal structures of T4, whose behavior in solution significantly differs beingness not comparable in dissimilar medium pH. The authors hypothesized that these changes in the pH-dependent solubility might bear on the oral availability and absorption of this drug (23). The overall aqueous solubility of levothyroxine sodium at 25°C decrease from medium pH 1 to 3, then reaching a nadir level until pH 7, level that stand for to a new increase of T4 solubility (24). The solubility is together with permeability the basis of the Biopharmaceutics Nomenclature System (BCS) (25). Based on the solubility and the permeability rates loftier or depression, drugs are in fact classified into one of four categories of the BCS. This has been proven difficult for levothyroxine sodium since in that location are sources classifying information technology as belonging to each of the abovementioned classes (26, 27). Interestingly, also the formation of large aggregates in aqueous media may enable the compound to attain concentration even higher than fifteen mg/100 ml (26).
Interference With Thyroxine Effectiveness Acting at Gastric Level
Nutrient and Drugs
Several drugs and foods accept been proven to interfere with the oral thyroxine absorption [run into for rev (6, 28, 29)]. The mechanisms described seem to touch each stride of oral and thyroxine absorption and metabolism and are chiefly exerted: a) by changing the gastric pH or adsorbing thyroxine in the breadbasket; b) by a possible competition with intestinal transporters or adsorbing thyroxine at the intestinal level; c) past affecting thyroxine binding on plasmatic proteins; d) past modulating catabolic thyroxine processes (half dozen, 28). The first two mechanisms are associated with an increased need for thyroxine and are shared by some interfering foods (6). Nutrient itself may represent a gastric hindrance to the bioavailability of drugs (xxx), including thyroxine (31). In clinical practice, the timing of food intake and the interval earlier and after thyroxine ingestion seems non negligible for the subsequent intestinal assimilation (31, 32).
As mentioned to a higher place, the mechanisms of interference affecting oral thyroxine during the gastric passage are substantially the variations of gastric juice pH and the bounden of thyroxine in an acidic environment. The antacids stand for one of the nigh prescribed categories of drugs worldwide: the interference with thyroxine bioavailability has been described for proton pump inhibitors and calcium carbonate (28). The outcome of proton pump inhibitors (PPI) seems to be related to their role in increasing gastric juice pH that might touch on disintegration and dissolution phases of tablet thyroxine (see for rev ref. 6), although their consequence on thyroxine absorption kinetics was denied by other authors (33, 34). Even so, the internet outcome of PPI on levothyroxine pharmacokinetic is more than complex and partially due to the circuitous variations of gastrointestinal physiology that may be restricted to the long-lasting apply of PPI (i.east. variations in gastric mucus viscosity, gastric and minor abdominal bacterial overgrowth) (35). Singh et al. (36) reported that both acute and chronic ingestions of calcium carbonate, as well as different preparations of calcium, are able to reduce the bioavailability of T4. Calcium carbonate showed a specific ability to bind thyroxine in vitro : indeed, it appears to bind thyroxine in a dose-dependent mode when medium pH is two; this binding disappears when the medium pH is 7.4, preventing absorption at the abdominal level (36). The negative impact of some nutrients on levothyroxine absorption has been demonstrated since 1977 (37). Virtually of nutrients (e.g. soy, fiber-enriched alimony and coffee, etc.) (6, 38, 39) specifically demark oral thyroxine at the intestinal level. Interestingly, still, some of them seem to interfere with thyroxine assimilation at gastric level like the fruit of papaya. The specific action of papaya seems to act fifty-fifty at gastric level since this fruit causes a significant reduction in histamine-induced acid secretion (40). Milk ingestion seems to interfere with thyroxine absorption for its poly peptide and calcium content as well as for its alkaline pH (41). Noticeably, nigh of antacid drugs may reduce the acidic exposure of thyroxine in the stomach merely they also adsorb the hormone in the upper abdominal tract (half-dozen).
Gastric Disorders and Surgical Procedures
From a clinical standpoint, the association between gastric and thyroid disorders is very frequent (42). An increased demand for thyroxine in patients with gastric disorders has been described in patients with Helicobacter pylori infection, chronic atrophic gastritis, in those who underwent gastric surgery or bearing gastroparesis. Amidst these, Helicobacter pylori infection is the most important since its prevalence has been estimated worldwide at 48%, despite wide regional discrepancies (Oceania 24% vs Africa 79%) (43). From its discovery in 1982 past Warren and Marshall, the role of Helicobacter pylori as cause of inflammatory gastritis in more of 90% of the cases has get clear (44). Commonly, Helicobacter pylori related gastritis initially involves the superficial layer of antrum mucosa of the breadbasket with an inflammatory mononuclear and plasma cells infiltrate. This stage of infection may characteristic an increased gastrin level and increased gastric juice acidity as well (45). Depending on cytotoxicity of bacterial strain and gastric environment characteristics, the degree of gastritis may get worsened up to atrophic pangastritis and intestinal metaplasia, determining hypo to achlorhydria (44). A role of Helicobacter pylori infection in impairing oral levothyroxine bioavailability was firstly described in 2006 (7). In this report and in the one past Bugdaci (46), the increased need for levothyroxine was reversed following H. pylori eradication. This latter paper also highlighted the possibility of iatrogenic thyrotoxicosis, maintaining the previous doses of thyroxine after the removal of infection (46). Undiagnosed or persistent H. pylori infection has been also proposed as a trigger for autoimmune atrophic gastritis (47, 48) through a molecular mimicry with epitopes of H+/1000+ATPase, the acid-producing pump of gastric parietal cells (48). In fact, autoimmune chronic gastritis shows a very high degree of corpus and fundus cloudburst of the breadbasket besides featuring positive autoantibodies against parietal cells and/or intrinsic factor (49, 50). This pathologic entity is frequently associated with autoimmune thyroid disorders (42, 51), being this association i of the most frequent cases of polyautoimmunity (42, 52). Thyroid and gastric autoimmune disorders are characterized by the action of environmental triggers on genetic predisposing background, leading to the loss of self-tolerance i.due east. of the residue between pro- and anti-inflammatory effector cells pathways (52, 53). The co-presence of thyroid and gastric autoimmune disorders features specific immunoregulatory cytokine profiles (54, 55). Autoimmune atrophic gastritis is characterized by achlorhydria and thus past a loftier oral levothyroxine requirement (7) being maximal in patients bearing the co-presence of gastric atrophy and Helicobacter pylori infection (7). The prevalence of autoimmune atrophic gastritis, which is often underdiagnosed, has been estimated as 0.v–v% (51). Achlorhydria is also a feature of laparoscopic sleeve gastrectomy (SG), the most mutual bariatric process performed in the USA (56, 57). The procedure implies the tubulization of the breadbasket betwixt fifty and 200 cc in volume while the remaining part of the stomach is removed (27). Despite nigh of the studies examining thyroxine requirement in SG patients described an unchanged or decreased dose of thyroxine needed by patients, the normalization by body weight conspicuously indicated an increased demand for the hormone following this bariatric procedure (56, 57). Patients undergoing bariatric surgery are oftentimes advised to utilize PPIs and micronutrients that may interfere with the assimilation of thyroxine; furthermore, their increased need for oral levothyroxine may be warranted past the variations in volume, acidic output, and motility of the remaining function of the stomach (27). These patients, in fact, often prove an acceleration of gastric elimination that may impair the disaggregation and dissolution of tablet levothyroxine (58). To note, an increased need for oral levothyroxine has been described in patients with the reverse motility disorder, i.e. gastroparesis (59, 60). Withal, its frequency is low and estimated in 9/100,000 men and 38/100,000 women (43).
How to Suspect Gastric Disorders Affecting Levothyroxine Absorption
Three main features may led to suspicion of a gastric disorder: clinical symptoms, malabsorption of drugs and micronutrients, and the presence of a chronic unexplained anemia (6). Despite the narrow therapeutic index, empiric and not targeted doses were widely used without proper label for long time (iii). On the contrary, an essential prerequisite to find gastric malabsorption is a careful tailoring of patient'southward treatment devoted to detect the minimal constructive dose of thyroxine (half dozen). Several characteristics of patients and their habits should be evaluated every bit shown in Figure 2. The timing of thyroxine ingestion represents a principal issue to obtain the therapeutic target using the lowest effective dose (vii, 31, 32, 61, 62). Other relevant characteristics are the lean body mass or the torso mass index, historic period, reproductive status, and the absence of bias. An accurate pharmacologic anamnestic investigation is, in fact, mandatory to avoid bias from widely used drugs and/or interfering foods (6, 28, 63, 64). In one case excluded all these putative biases, a gastrointestinal malabsorption of thyroxine may exist suspected (6). The concomitant presence of a macro- or microcytic anemia strengthens the hypothesis of a gastric problem (65, 66). A recent study observed that about one-half of patients with gastric cloudburst presented with anemia that was already severe at the time of diagnosis in one patient out of five (66). Atrophic gastritis is a prevalently silent disease that progresses from a mild chronic gastric inflammation to an advanced stage of atrophy and metaplasia (42). Anemia follows this worsening, proceeding from fe-deficient microcytic phenotype to vitamin B12 deficiency-associated macrocytic anemia (pernicious anemia) (65, 66). This latter is a issue of vitamin B12 malabsorption in turn due to intrinsic factor deficiency (66) whereas the reduced gastric acrid secretion lowers iron absorption in iron-deficient anemia (65). Some of these characteristics may prompt a screening for gastrointestinal disorders. The screening for these associated disorders has been recently described and reviewed (6).
Effigy two
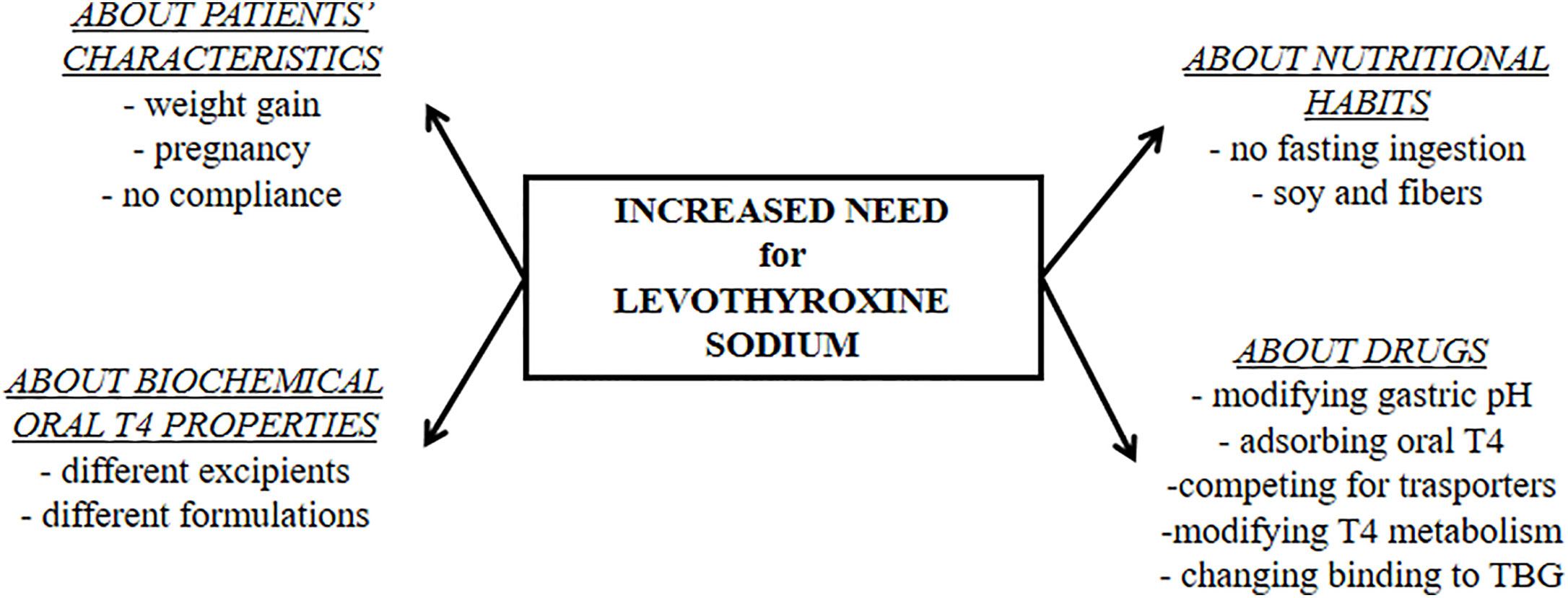
Figure 2 The physiologic and pharmacologic biases of oral levothyroxine treatment that must be excluded earlier starting a diagnostic workup for malabsorptive disorders.
At gastric level, the presence of specific antibodies confronting parietal cell and confronting H. pylori are reliable markers of suspicion as is for fasting gastrin levels. Notwithstanding, the diagnosis of superficial or atrophic gastritis must be based on multiple biopsies and histological examination (42).
The Use of Novel Conception in Thyroxine Increased Need Due to Gastric Disorders
The suboptimal efficiency of treatment worldwide (4) prompted manufacture to develop novel preparations of sodium levothyroxine. Recently, novel formulations of levothyroxine sodium accept been introduced: the soft gel capsules and the liquid solution (67). In the softgel capsules, levothyroxine is dissolved in glycerin and surrounded by a gelatin trounce while, in the liquid solution, the hormone is dissolved in 95% ethanol and 86% glycerol (Figure one).
A seminal in vitro study analyzed the dissolution at different medium pH of two tablet formulations (one brand and one generic) as compared to a softgel capsule (68). The latter performed ameliorate at medium pH >3, at which the dissolution bend of the levothyroxine sodium tablet clearly drops (24, 68). A pharmacokinetic report demonstrated that, in healthy subjects and in fasting conditions, softgel capsule formulation is bioequivalent to tablet thyroxine (69).
The dissolution fourth dimension of the softgel sheathing preparation has been directly observed during an endoscopy session in a healthy volunteer, demonstrating that the capsule completely disappeared 21 min post-obit its ingestion (70). Even when analyzed in patients bearing disorders or using drugs increasing gastric juice pH, the softgel formulation performed better than the traditional one (9).
The better performance of softgel conception in maintaining target TSH levels, despite a lower dose equally compared to the tablet 1, has been demonstrated in nigh of the patients bearing superficial gastritis, gastric atrophy, and resistant-to-handling Helicobacter pylori (71). Furthermore, ii case reports described patients begetting gastroparesis who benefited from the switching to softgel thyroxine to overcome the refractory hypothyroidism due to gastric motility impairment (72, 73)
The clinical efficacy of softgel formulation in a patient concomitantly treated with proton pump inhibitors has been described in a example-report (74). The better operation of softgel was confirmed past the indices of absorption, evaluated following an acute load with 600 mcg of thyroxine of the two formulations (74). Furthermore, the lesser touch on of concomitant breakfast ingestion on softgel sheathing preparation performance has been reported (75). To annotation, a study including patients with gastric disorders demonstrated that the switch from tablet to softgel levothyroxine causes a smaller number of dose adjustments, thus saving health costs (76).
The bioequivalence of the liquid thyroxine preparation to tablet thyroxine has been proven but, oving to the fact that the active ingredient is already dissolved, the time to achieve systemic circulation is significantly shorter as compared to both tablet and softgel preparations (77). Some case series reported the usefulness of this conception in pocket-sized grouping of patients with active H. pylori infection (78) or bearing atrophic gastritis (79). The liquid T4 formulation has been proven helpful also in the case of concomitant handling with proton pump inhibitors and several drugs with antacid activity (80, 81). A further relevant result is the effect of food co-ingestion on liquid thyroxine absorption: 2 papers agreed in defining this formulation less sensitive to the interfering activity of food when compared to the traditional i (82, 83). Noticeably, a study on more than fifty,000 thyroxine treated patients demonstrated a significant reduction in the number of serum TSH measurements after switching from tablet to liquid formulation (84). These results importantly pertain to patients using drugs interfering with levothyroxine absorption (84). Liquid formulation has been also proposed in a instance of sleeve gastrectomy (85). A recent meta-analysis on studies in which patients on tablet T4 showed suboptimal TSH values indicated that the switch to liquid T4 formulation, at the same daily dose, might help in reaching the target TSH levels (86). A further meta-assay reported no significant differences in patients without malabsorption just claimed that liquid thyroxine is more efficient than tablet L-T4 in treated patients with malabsorption (87).
Conclusions
Endocrinologists and physicians should be enlightened of the part of the stomach on the subsequent abdominal assimilation when treating patients with levothyroxine.
Author Contributions
CV and MC conceived of and designed the report. SC and NB performed the literature search. All authors contributed to the article and canonical the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or fiscal relationships that could be construed as a potential disharmonize of involvement.
References
one. Jonklaas J, Bianco AC, Bauer AJ, Burman KD, Cappola AR, Celi FS, et al. American Thyroid Association Task Force on Thyroid Hormone Replacement, Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association task force on thyroid hormone replacement. Thyroid (2014) 24:1670–751. doi: 10.1089/thy.2014.0028
PubMed Abstract | CrossRef Full Text | Google Scholar
3. Eligar 5, Taylor PN, Okosieme OE, Leese GP, Dayan CM. Thyroxine replacement: a clinical endocrinologist's viewpoint. Ann Clin Biochem (2016) 53(Pt4):421–33. doi: ten.1177/0004563216642255
PubMed Abstract | CrossRef Full Text | Google Scholar
v. Ernst FR, Barr P, Elmor R, Sandulli W, Thevathasan 50, Sterman AB, et al. The Economical Impact of Levothyroxine Dose Adjustments: the Command HE Study. Clin Drug Invest (2017) 37(i):71–83. doi: 10.1007/s40261-016-0462-3
CrossRef Full Text | Google Scholar
seven. Centanni 1000, Gargano L, Canettieri G, Viceconti N, Franchi A, Delle Fave G, et al. Thyroxine in goiter, Helicobacter pylori infection, and chronic gastritis. N Engl J Med (2006) 354:1787–95. doi: x.1056/NEJMoa043903
PubMed Abstract | CrossRef Total Text | Google Scholar
8. Sachmechi I, Reich DM, Aninyei Thousand, Wibowo F, Gupta G, Kim PJ. Effect of proton pump inhibitors on serum thyroid-stimulating hormone level in euthyroid patients treated with levothyroxine for hypothyroidism. Endocr Pract (2007) 13(4):345–9. doi: x.4158/EP.13.4.345
PubMed Abstract | CrossRef Full Text | Google Scholar
9. Seng Yue C, Benvenga S, Scarsi C, Loprete L, Ducharme MP. When Bioequivalence in Healthy Volunteers May non Translate to Bioequivalence in Patients: Differential Effects of Increased Gastric pH on the Pharmacokinetics of Levothyroxine Capsules and Tablets. J Pharm Pharm Sci (2015) eighteen(v):844–55. doi: ten.18433/j36p5m
PubMed Abstruse | CrossRef Full Text | Google Scholar
10. Chemburkar SR, Deming KC, Reddy RE. Chemistry of thyroxine: an historical perspective and recent progress on its synthesis. Tetrahedron (2010) 66:1955–62. doi: 10.1016/j.tet.2009.12.044
CrossRef Full Text | Google Scholar
13. Stewart KD, Johnston JA, Matza LS, Curtis SE, Havel HA, Sweetana SA, et al. Preference for pharmaceutical formulation and treatment process attributes. Patient Prefer Adherence (2016) ten:1385–99. doi: 10.2147/PPA.S101821
PubMed Abstract | CrossRef Full Text | Google Scholar
16. Van Den Abeele J, Rubbens J, Brouwers J, Augustijns P. The dynamic gastric surround and its affect on drug and formulation behaviour. Eur J Pharm Sci (2017) 96:207–31. doi: 10.1016/j.ejps.2016.08.060
PubMed Abstract | CrossRef Total Text | Google Scholar
17. Hogben CA, Schanker LS, Tocco DJ, Brodie BB. Absorption of drugs from the tum. Ii. The human being. J Pharmacol Exp Ther (1957) 120(iv):540–5.
PubMed Abstract | Google Scholar
xix. Hörter D, Dressman JB. Influence of physicochemical properties on dissolution of drugs in the gastrointestinal tract. Adv Drug Deliv Rev (2001) 46:75–87. doi: ten.1016/s0169-409x(00)00130-7
PubMed Abstract | CrossRef Full Text | Google Scholar
20. Jambhekar SS, Breen PJ. Drug dissolution: significance of physicochemical properties and physiological conditions. Drug Discov Today (2013) 18(23-24):1173–84. doi: 10.1016/j.drudis.2013.08.013
PubMed Abstract | CrossRef Full Text | Google Scholar
21. Mitra A, Kesisoglou F. Impaired drug absorption due to loftier stomach pH: a review of strategies for mitigation of such consequence to enable pharmaceutical product evolution. Mol Pharm (2013) 10(11):3970–9. doi: 10.1021/mp400256h
PubMed Abstract | CrossRef Total Text | Google Scholar
25. Lindenberg M, Kopp S, Dressman JB. Classification of orally administered drugs on the Earth Health Organization model list of essential medicines co-ordinate to the Biopharmaceutics Classification Organisation. Eur J Pharm Biopharm (2004) 58:265–78. doi: ten.1016/j.ejpb.2004.03.001
PubMed Abstract | CrossRef Full Text | Google Scholar
26. Kocic I, Homsek I, Dacevic K, Parojcic J, Miljkovic B. An investigation into the influence of experimental conditions on in vitro drug release from immediate-release tablets of levothyroxine sodium and its relation to oral bioavailability. AAPS PharmSciTech (2011) 12(3):938–48. doi: 10.1208/s12249-011-9660-viii
PubMed Abstruse | CrossRef Full Text | Google Scholar
27. Azran C, Porat D, Fine-Shamir N, Hanhan N, Dahan A. Oral levothyroxine therapy postbariatric surgery: Biopharmaceutical aspects and clinical effects. Surg Obes Relat Dis (2019) xv(2):333–41. doi: 10.1016/j.soard.2019.01.001
PubMed Abstract | CrossRef Full Text | Google Scholar
29. Colucci P, Yue CS, Ducharme One thousand, Benvenga South. A Review of the Pharmacokinetics of Levothyroxine for the Treatment of Hypothyroidism. Eur Endocrinol (2013) 9(1):40–seven. doi: ten.17925/EE.2013.09.01.40
PubMed Abstract | CrossRef Full Text | Google Scholar
31. Bach-Huynh TG, Nayak B, Loh J, Soldin S, Jonklaas J. Timing of levothyroxine administration affects serum thyrotropin concentration. J Clin Endocrinol Metab (2009) 94:3905–12. doi: 10.1210/jc.2009-0860
PubMed Abstract | CrossRef Full Text | Google Scholar
32. Centanni M, Benvenga S, Sachmechi I. Diagnosis and management of handling-refractory hypothyroidism: an skilful consensus report. J Endocrinol Invest (2017) twoscore(12):1289–301. doi: x.1007/s40618-017-0706-y
PubMed Abstract | CrossRef Full Text | Google Scholar
33. Dietrich JW, Gieselbrecht K, Holl RW, Boehm BO. Absorption kinetics of levothyroxine is not altered by proton-pump inhibitor therapy. Horm Metab Res (2006) 38(1):57–ix. doi: 10.1055/s-2006-924980
PubMed Abstract | CrossRef Full Text | Google Scholar
34. Ananthakrishnan Due south, Braverman LE, Levin RM, Magnani B, Pearce EN. The effect of famotidine, esomeprazole, and ezetimibe on levothyroxine assimilation. Thyroid (2008) 18(5):493–8. doi: 10.1089/thy.2007.0381
PubMed Abstruse | CrossRef Full Text | Google Scholar
35. Goddard AF, Spiller RC. The effect of omeprazole on gastric juice viscosity, pH and bacterial counts. Aliment Pharmacol Ther (1996) 10(1):105–ix. doi: x.1111/j.1365-2036.1996.tb00183.10
PubMed Abstract | CrossRef Full Text | Google Scholar
38. Pinchera A, Macgillivray MH, Crawford JD, Freeman AG. Thyroid refractoriness in an athyreotic cretin fed soybean formula. Due north Engl J Med (1965) 273:83–7. doi: x.1056/NEJM196507082730205
PubMed Abstruse | CrossRef Full Text | Google Scholar
39. Benvenga S, Bartolone L, Pappalardo MA, Russo A, Lapa D, Giorgianni G, et al. Altered intestinal absorption of L-thyroxine caused by coffee. Thyroid (2008) 18(3):293–301. doi: 10.1089/thy.2007.0222
PubMed Abstract | CrossRef Full Text | Google Scholar
40. Deiana L, Marini S, Mariotti S. Ingestion of large amounts of papaya fruit and impaired effectiveness of levothyroxine therapy. Endocr Pract (2012) 18(one):98–100. doi: x.4158/EP11233.CO
PubMed Abstruse | CrossRef Full Text | Google Scholar
42. Lahner E, Conti L, Cicone F, Capriello S, Cazzato M, Centanni Grand, et al. Thyro-entero-gastric autoimmunity: Pathophysiology and implications for patient management. Best Pract Res Clin Endocrinol Metab (2020) 34(i):101373. doi: x.1016/j.beem.2019.101373
PubMed Abstract | CrossRef Full Text | Google Scholar
43. Castellana M, Castellana C, Giovanella Fifty, Trimboli P. Prevalence of gastrointestinal disorders having an impact on tablet levothyroxine absorption: should this formulation nonetheless be considered as the beginning-line therapy? Endocrine (2020) 67(ii):281–90. doi: 10.1007/s12020-019-02185-iv
PubMed Abstract | CrossRef Full Text | Google Scholar
44. Sipponen P, Maaroos HI. Chronic gastritis. Scand J Gastroenterol (2015) 50:6, 657–667. doi: 10.3109/00365521.2015.1019918
CrossRef Full Text | Google Scholar
45. Sachs G, Shin JM, Munson K, Vagin O, Lambrecht N, Scott DR, et al. The command of gastric acid and Helicobacter pylori eradication. Aliment Pharmacol Ther (2000) 14:1383–401. doi: 10.1046/j.1365-2036.2000.00837.10
PubMed Abstract | CrossRef Full Text | Google Scholar
46. Bugdaci MS, Zuhur SS, Sokmen M, Toksoy B, Bayraktar B, Altuntas Y. The role of Helicobacter pylori in patients with hypothyroidism in whom could non be achieved normal thyrotropin levels despite treatment with high doses of thyroxine. Helicobacter (2011) 16(2):124–30. doi: ten.1111/j.1523-5378.2011.00830.ten
PubMed Abstract | CrossRef Full Text | Google Scholar
47. Kuipers EJ, Uyterlinde AM, Peña As, Roosendaal R, Pals Grand, Nelis GF, et al. Long-term sequelae of Helicobacter pylori gastritis. Lancet (1995) 345:1525–viii. doi: ten.1016/S0140-6736(95)91084-0
PubMed Abstract | CrossRef Full Text | Google Scholar
48. D'Elios MM, Bergman MP, Azzurri A, Amedei A, Benagiano Thou, De Pont JJ, et al. H+/K+-ATPase (proton pump) is the target autoantigen of Th1-type cytotoxic T cells in autoimmune gastritis. Gastroenterology (2001) 120:377–86. doi: 10.1053/gast.2001.21187
PubMed Abstract | CrossRef Full Text | Google Scholar
49. Neumann WL, Coss E, Rugge M, Genta RM. Autoimmune atrophic gastritis–pathogenesis, pathology and management. Nat Rev Gastroenterol Hepatol (2013) x:529–4. doi: 10.1038/nrgastro.2013.101
PubMed Abstract | CrossRef Full Text | Google Scholar
51. Lahner Due east, Intraligi Thousand, Buscema Thou, Centanni M, Vannella L, Grossi E, et al. Artificial Neural Networks in the recognition of the presence of thyroid illness in patients with atrophic body gastritis. World J Gastroenterol (2008) xiv:563–8. doi: 10.3748/wjg.14.563
PubMed Abstract | CrossRef Full Text | Google Scholar
52. Bliddal S, Nielsen CH, Feldt-Rasmussen U. Recent advances in agreement autoimmune thyroid illness: the tallest tree in the forest of polyautoimmunity. F1000Res (2017) 6:1776. doi: 10.12688/f1000research.11535.1
PubMed Abstract | CrossRef Full Text | Google Scholar
54. Santaguida MG, Nardo S, Del Duca SC, Lococo Due east, Virili C, Gargano L, et al. Increased interleukin-4-positive lymphocytes in patients with Hashimoto'south thyroiditis and concurrent not-endocrine autoimmune disorders. Clin Exp Immunol (2011) 165:148–54. doi: 10.1111/j.1365-2249.2011.04419.10
PubMed Abstruse | CrossRef Full Text | Google Scholar
55. Santaguida MG, Gatto I, Mangino G, Virili C, Stramazzo I, Fallahi P, et al. BREG cells in Hashimoto'southward thyroiditis isolated or associated to farther organ-specific autoimmune diseases. Clin Immunol (2017) 184:42–vii. doi: 10.1016/j.clim.2017.04.012
PubMed Abstract | CrossRef Full Text | Google Scholar
56. Pedro J, Cunha F, Souteiro P, Neves JS, Guerreiro Five, Magalhães D, et al. The Issue of the Bariatric Surgery Type on the Levothyroxine Dose of Morbidly Obese Hypothyroid Patients. Obes Surg (2018) 28:3538–43. doi: x.1007/s11695-018-3388-iv
PubMed Abstruse | CrossRef Full Text | Google Scholar
58. Vigneshwaran B, Wahal A, Aggarwal South, Priyadarshini P, Bhattacharjee H, Khadgawat R, et al. Bear on of Sleeve Gastrectomy on Type ii Diabetes Mellitus, Gastric Emptying Time, Glucagon-Like Peptide 1 (GLP-1), Ghrelin and Leptin in Non-morbidly Obese Subjects with BMI thirty-35.0 kg/mtwo: a Prospective Report. Obes Surg (2016) 26(12):2817–23. doi: 10.1007/s11695-016-2226-9
PubMed Abstruse | CrossRef Total Text | Google Scholar
59. Khraisha Bone, Al-Madani MM, Peiris AN, Paul TK. Gastroparesis - a novel cause of persistent thyroid stimulating hormone elevation in hypothyroidism. J La State Med Soc (2015) 167(2):47–9.
PubMed Abstract | Google Scholar
61. Bolk N, Visser TJ, Nijman J, Jongste IJ, Tijssen JG, Berghout A. Effects of evening vs morning levothyroxine intake: a randomized double-bullheaded crossover trial. Arch Intern Med (2010) 170(22):1996–2003. doi: 10.1001/archinternmed.2010.436
PubMed Abstruse | CrossRef Total Text | Google Scholar
64. Skelin Chiliad, Lucijanić T, Amidžić Klarić D, Rešić A, Bakula M, Liberati-Čizmek AM, et al. Factors Affecting Gastrointestinal Absorption of Levothyroxine: A Review. Clin Ther (2017) 39(2):378–403. doi: 10.1016/j.clinthera.2017.01.005
PubMed Abstract | CrossRef Full Text | Google Scholar
65. Sibilla R, Santaguida MG, Virili C, Gargano L, Nardo South, Della Guardia One thousand, et al. Chronic unexplained anemia in isolated autoimmune thyroid disease or associated with autoimmune related disorders. Clin Endocrinol (Oxf) (2008) 68:640–5. doi: x.1111/j.1365-2265.2007.03091.x
PubMed Abstract | CrossRef Total Text | Google Scholar
66. Lenti MV, Lahner E, Bergamaschi G, Miceli East, Conti Fifty, Massironi Due south, et al. Cell Claret Count Alterations and Patterns of Anaemia in Autoimmune Atrophic Gastritis at Diagnosis: A Multicentre Study. J Clin Med (2019) eight(11):1992. doi: 10.3390/jcm8111992
CrossRef Full Text | Google Scholar
68. Pabla D, Akhlaghi F, Zia H. A comparative pH-dissolution profile study of selected commercial levothyroxine products using inductively coupled plasma mass spectrometry. Eur J Pharm Biopharm (2009) 72:105–10. doi: ten.1016/j.ejpb.2008.10.008
PubMed Abstract | CrossRef Full Text | Google Scholar
69. Colucci P, D'Angelo P, Mautone Thou, Scarsi C, Ducharme MP. Pharmacokinetic Equivalence of a Levothyroxine Sodium Soft Capsule Manufactured Using the New Food and Drug Assistants Potency Guidelines in Healthy Volunteers Nether Fasting Conditions. Ther Drug Monit (2011) 33(3):355–61. doi: 10.1097/FTD.0b013e318217b69f
PubMed Abstract | CrossRef Total Text | Google Scholar
71. Santaguida MG, Virili C, Del Duca SC, Cellini 1000, Gatto I, Brusca N, et al. Thyroxine softgel capsule in patients with gastric-related T4 malabsorption. Endocrine (2015) 49:51–seven. doi: x.1517/17425247.2014.918101
PubMed Abstruse | CrossRef Full Text | Google Scholar
72. Kim PJ, Sachmechi I. Levothyroxine malabsorption induced by diabetic gastroparesis exacerbated during pregnancies: outcome of intramuscular levothyroxine injections and levothyroxine softgel capsules. AACE Clin Case Rep (2015) 1:e73–8. doi: x.4158/EP14051.CR
CrossRef Full Text | Google Scholar
73. Reardon DP, Yoo PS. Levothyroxine tablet malabsorption associated with gastroparesis corrected with gelatin capsule formulation. Case Rep Endocrinol (2016) 2016:1316724. doi: 10.1155/2016/1316724
PubMed Abstract | CrossRef Full Text | Google Scholar
74. Vita R, Benvenga Due south. Tablet levothyroxine (L-T4) malabsorption induced by proton pump inhibitor; a problem that was solved by switching to 50-T4 in soft gel capsule. Endocr Pract (2014) 20:e38–41. doi: 10.4158/EP13316.CR
PubMed Abstract | CrossRef Full Text | Google Scholar
75. Cappelli C, Pirola I, Gandossi E, Cristiano A, Daffini L, Agosti B, et al. Thyroid hormone profile in patients ingesting softgel capsule or liquid levothyroxine formulations with breakfast. Int J Endocrinol (2016) 2016:9043450. doi: 10.1155/2016/9043450
PubMed Abstract | CrossRef Total Text | Google Scholar
76. Ernst FR, Sandulli Due west, Elmor R, Welstead J, Sterman AB, Lavan M. Retrospective study of patients switched from tablet formulations to a gel cap formulation of levothyroxine: results of the CONTROL Switch Written report. Drugs R D (2017) 17:103–15. doi: 10.1007/s40268-016-0150-z
PubMed Abstract | CrossRef Total Text | Google Scholar
77. Yue CS, Scarsi C, Ducharme MP. Pharmacokinetics and potential advantages of a new oral solution of levothyroxine vs. other available dosage forms. Arzneimittelforschung (2012) 62:631–6. doi: x.1055/s-0032-1329951
PubMed Abstract | CrossRef Full Text | Google Scholar
78. Ribichini D, Fiorini G, Repaci A, Castelli V, Gatta L, Vaira D, et al. Tablet and oral liquid 50-thyroxine formulation in the treatment of naïve hypothyroid patients with Helicobacter pylori infection. Endocrine (2017) 57:394–401. doi: x.1007/s12020-016-1167-3
PubMed Abstract | CrossRef Total Text | Google Scholar
79. Fallahi P, Ferrari SM, Ruffilli I, Antonelli A. Reversible normalisation of serum TSH levels in patients with autoimmune atrophic gastritis who received Fifty-T4 in tablet form after switching to an oral liquid formulation: a instance series. BMC Gastroenterol (2016) 16:22. doi: ten.1186/s12876-016-0439-y
PubMed Abstract | CrossRef Full Text | Google Scholar
eighty. Vita R, Saraceno Thou, Trimarchi F, Benvenga South. Switching levothyroxine from the tablet to the oral solution conception corrects the impaired absorption of levothyroxine induced by proton-pump inhibitors. J Clin Endocrinol Metab (2014) 99(12):4481–vi. doi: 10.1210/jc.2014-2684
PubMed Abstract | CrossRef Full Text | Google Scholar
81. Vita R, Di Bari F, Benvenga S. Oral liquid levothyroxine solves the problem of tablet levothyroxine malabsorption due to concomitant intake of multiple drugs. Skilful Opin Drug Deliv (2017) 14:467–72. doi: 10.1080/17425247.2017.1290604
PubMed Abstruse | CrossRef Full Text | Google Scholar
82. Cappelli C, Pirola I, Daffini 50, Formenti A, Iacobello C, Cristiano A, et al. A double-blind placebo-controlled trial of liquid thyroxine ingested at breakfast: results of the TICO report. Thyroid (2016) 26:197–202. doi: 10.1089/thy.2015.042234
PubMed Abstruse | CrossRef Total Text | Google Scholar
83. Morelli S, Reboldi G, Moretti Southward, Menicali E, Avenia N, Puxeddu E. Timing of breakfast does non influence therapeutic efficacy of liquid levothyroxine formulation. Endocrine (2015) 52:571–viii. doi: 10.1007/s12020-015-0788-two
PubMed Abstract | CrossRef Full Text | Google Scholar
84. Ferrara R, Ientile 5, Arcoraci V, Ferrajolo C, Piccinni C, Fontana A, et al. Treatment pattern and frequency of serum TSH measurement in users of different levothyroxine formulations: a population-based study during the years 2009-2015. Endocrine (2017) 58(1):143–52. doi: 10.1007/s12020-017-1242-iv
PubMed Abstract | CrossRef Full Text | Google Scholar
85. Hommel C, Delgrange E. Resistance to levothyroxine in a bariatric surgery patient: an indication for liquid formulation? Acta Clin Belg (2017) 72(ane):72–5. doi: x.1080/17843286.2016.1196861
PubMed Abstract | CrossRef Full Text | Google Scholar
86. Virili C, Giovanella L, Fallahi P, Antonelli A, Santaguida MG, Centanni M, et al. Levothyroxine Therapy: Changes of TSH Levels past Switching Patients from Tablet to Liquid Conception. A Systematic Review and Meta-Analysis. Front Endocrinol (Lausanne) (2018) 9:x. doi: ten.3389/fendo.2018.00010
PubMed Abstract | CrossRef Full Text | Google Scholar
87. Laurent I, Tang S, Astère M, Wang KR, Deng South, Xiao 50, et al. Liquid L-thyroxine versus tablet L-thyroxine in patients on L- thyroxine replacement or suppressive therapy: a meta-assay. Endocrine (2018) 61(1):28–35. doi: x.1007/s12020-018-1574-viii
PubMed Abstract | CrossRef Full Text | Google Scholar
Source: https://www.frontiersin.org/articles/10.3389/fendo.2020.621616/full
0 Response to "Easy on the Stomach Medications to Treat Thyroid Conditions"
Post a Comment